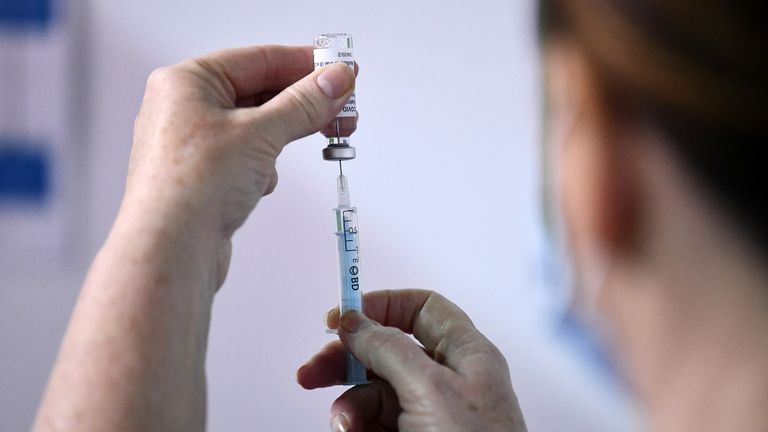
AstraZeneca has begun the worldwide withdrawal of its COVID vaccine – ending an era in which it saved millions of lives while being dogged by controversy.
The jab, developed at Oxford University, was once hailed as “the vaccine for the world” because it could be stored in fridges rather than the ultra-cold freezer temperatures originally required for alternative mRNA shots, making it more suitable for developing countries.
But AstraZeneca (AZ) said demand for the jab had tailed off as more up-to-date vaccines target newer variants of the virus. It no longer manufactures nor supplies the vaccine.
The voluntary withdrawal has started in the EU, with the European Medicines Agency announcing that the vaccine is no longer authorised for use.
In a statement, AZ said: “According to independent estimates, over 6.5 million lives were saved in the first year of use alone and over three billion doses were supplied globally.
“Our efforts have been recognised by governments around the world and are widely regarded as being a critical component of ending the global pandemic.
“We will now work with regulators and our partners to align on a clear path forward to conclude this chapter and significant contribution to the COVID-19 pandemic.”
The vaccine had only been in use for a few months before reports emerged of unusual blood clots.
Thrombosis with thrombocytopenia, or TTS, occurred in around one in 50,000 patients and was sometimes fatal.
Several European countries and some Canadian provinces suspended use of the vaccine as a result.
In the UK, medicines regulators responded by first recommending the vaccine only for people over 30, before raising the age cut-off to 40.
The vaccine hasn’t been used in the UK since the start of the booster programme, though the government says this was because mRNA vaccines were more effective, not because of concern over side effects.
However, more than 50 people who developed clots after vaccination are taking legal action against AZ in the High Court, claiming that the company failed to adequately inform them of a potential risk.
The company says the clinical trials and real-world evidence have shown the vaccine has “an adequate safety profile”.